Introduction
In the vast realm of scientific exploration, the concept of mass holds tremendous significance. Mass, in its essence, refers to the amount of matter contained within an object. It plays a pivotal role in various scientific disciplines, providing insights into the fundamental nature of our universe. This article delves into the intriguing world of mass, tracing its historical development, exploring its fundamental properties, discussing its units and measurements, and highlighting its applications in different fields of science.
Historical Development of the Concept of Mass
Throughout history, the understanding of mass has evolved hand in hand with scientific progress. In ancient times, mass was primarily associated with the heaviness or lightness of objects. However, it was the pioneering work of Sir Isaac Newton that laid the foundation for our modern understanding of mass. Newton’s laws of motion introduced the concept of inertia, which revealed the intrinsic property of mass that resists changes in motion.
Advancements in measurement techniques further propelled the study of mass. The invention of the balance scale, which compares the mass of an unknown object with a known mass, revolutionized the field. Over time, more precise instruments and methodologies emerged, leading to accurate measurements of mass in scientific experiments.
Fundamental Properties of Mass
Inertia, one of the fundamental properties of mass, refers to the tendency of an object to maintain its state of motion. The greater an object’s mass, the stronger its inertia, and the more force is required to accelerate or decelerate it.
Mass also plays a crucial role in the phenomenon of gravity. According to Newton’s law of universal gravitation, the gravitational force between two objects is directly proportional to their masses and inversely proportional to the square of the distance between them. Thus, the mass of an object determines the strength of its gravitational pull.
Another important concept related to mass is the conservation of mass. In a closed system, mass remains constant over time, regardless of any physical or chemical changes that may occur. This principle serves as a cornerstone in various scientific fields, including chemistry and thermodynamics.
Units of Mass
In the realm of scientific measurement, the International System of Units (SI) provides a standardized framework for expressing mass. The base unit for mass in the SI system is the kilogram (kg), which is defined as the mass of the International Prototype of the Kilogram.
While the kilogram is the primary unit, other commonly used mass units include grams (g) and metric tons (t). Converting between these units involves simple multiplication or division by factors of ten, as the SI system follows a decimal-based structure.
Mass vs. Weight
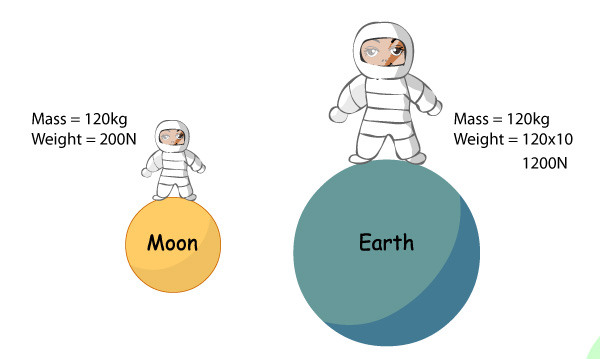
While mass and weight are often used interchangeably in everyday language, they have distinct scientific meanings. Mass refers to the amount of matter in an object, whereas weight measures the force exerted by gravity on that object. Weight depends not only on mass but also on the strength of the gravitational field.
For example, an object with a mass of 1 kilogram on Earth would have a weight of approximately 9.8 newtons due to the acceleration of gravity. However, if the same object were taken to the Moon, where the gravitational pull is weaker, its weight would be significantly less.
Mass in Particle Physics
In the realm of particle physics, the concept of mass takes on a unique dimension. Subatomic particles, such as protons, neutrons, and electrons, possess mass. Understanding the masses of these particles is crucial for unraveling the complexities of the quantum world.
Furthermore, the concept of mass-energy equivalence, famously represented by Einstein’s equation E=mc², reveals that mass and energy are interchangeable. This groundbreaking insight has profound implications for our understanding of the universe and forms the basis of nuclear energy.
Applications of Mass in Science
Mass finds practical applications in various scientific disciplines. In astrophysics, the study of celestial bodies relies heavily on measuring the masses of stars, planets, and galaxies. These measurements help scientists understand the formation, evolution, and dynamics of the universe.
In the field of chemistry, mass spectrometry plays a crucial role. This analytical technique enables the determination of the mass-to-charge ratio of ions, aiding in the identification and characterization of chemical compounds.
Mass is also integral to engineering and mechanics, where it influences the design and behavior of structures and machines. Understanding the mass distribution and its impact on stability, vibrations, and motion is essential for creating efficient and safe engineering solutions.
Challenges in Measuring Mass
Measuring mass with precision and accuracy poses significant challenges. Environmental influences, such as air currents and temperature fluctuations, can affect measurements. To mitigate these factors, scientists employ techniques such as using vacuum chambers or employing shields to isolate the object being measured.
Sophisticated instruments, including electronic balances and mass spectrometers, have been developed to achieve highly accurate mass measurements. These instruments rely on principles such as electromagnetic force compensation or time-of-flight analysis to determine mass with utmost precision.
Mass and the Higgs Boson
The discovery of the Higgs Boson, a particle associated with the Higgs field, revolutionized our understanding of mass in the context of particle physics. The Higgs field permeates the universe and interacts with particles, endowing them with mass. This interaction is instrumental in explaining why certain particles have mass while others do not.
The Higgs Boson discovery at CERN’s Large Hadron Collider confirmed the existence of the Higgs field and provided experimental evidence for the mechanism by which particles acquire mass. This breakthrough deepened our understanding of the fundamental nature of matter and energy.
Conclusion
In conclusion, mass is a fundamental concept in the realm of science, serving as a cornerstone in various scientific disciplines. It has a rich historical development, rooted in ancient observations and refined through centuries of scientific progress. The fundamental properties of mass, its units of measurement, and its applications across diverse fields exemplify its importance.
From particle physics to astrophysics, from chemistry to engineering, mass underpins our understanding of the natural world. As scientific exploration continues, the exploration of mass and its intricacies will undoubtedly lead to new discoveries, further expanding our understanding of the universe we inhabit.